Hydrochlorofluorocarbons (HCFCs) are man-made chemical compounds commonly used in the foam, refrigeration, and air conditioning sector. Unfortunately, recent studies have shown that HCFCs destroy the protective ozone layer and contribute to climate change, according to the United Nations Industrial Development Organization (UNIDO).
Because of this, the government is pushing for the establishment of policies for the use of “low carbon, energy efficient” cold chain systems to totally eliminate the use of HCFCs in the country, a press statement said.
In the $27.5 project called the “Global Partnership for Improving the Food Cold Chain in the Philippines” (GPI-FCCP), the Department of Environment and Natural Resources (DENR) is also carrying out a program putting up environment-friendly cold chain facilities.
The press statement said the country’s refrigeration systems for transporting goods for the food industry will no longer use ozone-depleting substances (ODS) like HCFC. Stringent policies are important in providing a stable investment environment for investors in “green” cooling technologies, the DENR said.
Cold chain covers every product that needs cooling from the “field to the fork (transport, storage, transformation, packaging). Policies will involve national standards for flammable refrigerants and revision of energy efficiency standards.
The project is assisted by international funder Global Environment Facility (GEF). Among the initiatives it will conduct include training of 200 key stakeholders on energy-efficiency and climate-friendly cold chain technologies. There is also a high level training for 50 local engineers, system suppliers and end-users on the use of global innovative cold chain technology.
The environment department said the cold chain project came about as part of the country’s compliance to its commitment to the 1987 Montreal Protocol, a global agreement to protect the stratospheric ozone layer by phasing out the production and consumption of ODS.
The Montreal Protocol compelled signatory countries to freeze consumption and production of the HCFCs. Developing countries should have cut by 100% their HCFC production by 2030.
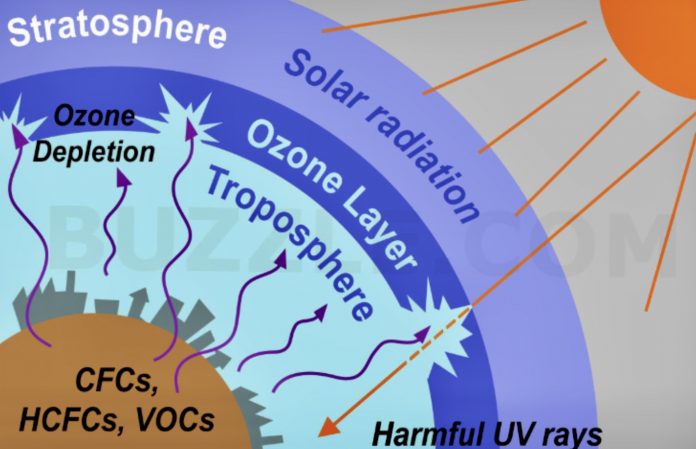
The earth’s atmosphere is divided into several layers. According to science, the lower region – called troposphere – extends from the planet’s surface up to about 10 kilometers in altitude. (For the uninformed, Mount Everest, the tallest mountain on the planet, is only about 9 kilometers high.) All human activities occur in this layer.
Ozone at the ground level, at the bottom of the troposphere is a harmful pollutant. One of the greenhouse gases, it is actually a result from the exhausts of automobiles and other vehicles and other sources.
After the troposphere comes the stratosphere, which continues from 10 kilometers to about 50 kilometers. Most commercial airline traffic occurs in the lower part of the stratosphere. It is in this layer where the ozone shield is found.
“If all the ozone contained in the atmosphere from ground level to a height of some 50 kilometers could be assembled at the earth’s surface, it would comprise a layer of gas only about three millimeters thick, weighing some 3,000 million tons,” a science scribe wrote.
Why is there so much ado about the ozone layer? Nothing except that the existence of humans and other terrestrial beings depends on the presence of ozone. Studies have shown that depletion of the ozone layer would allow more ultraviolet radiation to reach the earth’s surface.
“Protecting the ozone is crucial since it is the only gas in the atmosphere that limits the amount of harmful solar ultraviolet radiation reaching the earth,” points out Cynthia Pollack Shea, a senior researcher at the Washington, D.C.-based Worldwatch Institute. “Without ozone, life on earth would be impossible.”
The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. “Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature of 5,500-6,000 K (5,230-5,730⁰C) except that there was no radiation below a wavelength of about 310 nanometers at the ultraviolet end of the spectrum,” Wikipedia states.
It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually, the spectrum of the missing radiation was matched to only one known chemical, ozone.
The properties of ozone were explored in detail by the British meteorologist G.M.B. Dobson, who developed a simple spectrophotometer that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day.
Then, it was found that the ozone layer was thinning out. This was observed when a British Antarctic survey headed by Dr. Joseph Farman monitored ozone levels from Halley Bay, a scientific outpost on the Antarctic coast in 1957. Measurements taken with the spectrophotometer remained stable until the team observed in the 1970s a sharp decline in ozone during the months of September and October.
At first, the British scientists refused to believe their findings, dismissing them as either human or technical error. The 40% drop was too significant to be believed. The “hole,” as the media called it, was as big as the United States and as deep as Mount Everest.
In 1974, two American scientists – Drs. Mario Molina and F. Sherwood Rowland from the Jet Propulsion Institute of Pasadena and the University of Southern California in Irvine, respectively – hypothesized that man-made chlorofluorocarbons (CFCs) were escaping into the atmosphere and “eating” the ozone layer.
In 1976, it was found that the concentration of chlorine in the atmosphere – which were released from CFCs – was 1.25 parts per billion (ppb). The number doubled in 1989.
“Although the figures may sound like a ridiculously small amount, CFCs are about 15,000 times more efficient at producing greenhouse effect. So, a little means a lot. About 20% of global warming is attributed to CFCs,” wrote H. Steven Dashfhersky, author of the book, Environmental Literacy.
It was then that scientists became alarmed as the effects could be lethal. UVB (the higher energy ultraviolet radiation absorbed by ozone) is generally accepted to be a contributory factor to skin cancer and to produce Vitamin D. In addition, increased surface ultraviolet leads to increased tropospheric ozone, which is a health risk to humans.
Another form of skin cancer – malignant melanoma – is much less common but far more dangerous, being lethal in about 15–20% of the cases diagnosed. The relationship between malignant melanoma and ultraviolet exposure is not yet well understood, but it appears that both UVB and UVA are involved.
There are also studies suggesting the association between ocular cortical cataracts and UVB exposure. In the Philippines, 400,000 Filipinos get blind because of cataract, whose global cost of blindness is approximately 25 billion dollars annually in lost productivity.
“When a cataract happens, the amount of light that can enter the eye is reduced and the patient complains of blurring of vision especially in bright sunlight when the pupil gets constricted and also glare at night when driving,” says Dr. Manuel Agulto, chair of the Department of Ophthalmology at the University of the Philippines College of Medicine. “These complaints are progressive and by the time the cataract has become matured (noticeable by its white color) the patient is already blind.”
Ozone depletion can also have devastating effects on the environment. According to the US Environmental Protection Agency (EPA), depletion of the ozone layer could reduce crop yields and seriously disturb the balance of the ecosystems of the oceans.
Had the depletion of the ozone layer continued, the world would be at least 25% hotter today. “That additional heat energy would have provided ‘fuel’ for today’s extreme weather events like typhoons, floods and droughts,” said Dr. Rolando Garcia, a senior scientist at the US National Center for Atmospheric Research.
In 1987, the Montreal Protocol was created to reduce the amounts of ozone-depleting substances like CFCs, halons (used in firefighting equipment) and solvents like carbon tetrachloride and methyl chloroform.
Thanks to the said protocol, the ozone layer is expected to recover by 2050, according to recent research done by the University of Leeds and Massachusetts Institute of Technology. “But the Protocol has two major pieces of unfinished business,” wrote Stephen Leahy in a news report published by the National Geographic. “Some countries in the developing world haven’t yet phased out ozone-damaging chemicals like R-22, a hydrochlorofluorocarbon found in many refrigeration and air conditioning systems.” (Photo from publichealthnotes.com)